The Future of Electric Vehicle Batteries
The electric vehicle (EV) industry continues to demand batteries that are safer, denser in energy, and capable of longer life cycles to meet rising consumer expectations and environmental policies. Solid-state batteries (SSBs) are considered a promising solution due to their inherent safety and energy density advantages over traditional lithium-ion batteries. However, developing solid electrolytes that operate at high voltages while retaining stability has been a critical barrier. Recently, researchers at Yonsei University pioneered a new fluoride-based solid electrolyte that safely surpasses the 5-volt threshold, a milestone that could transform EV battery technology and large-scale renewable energy storage. This advancement addresses key challenges in battery chemistry and opens the door to more robust, cost-effective, and high-performance electric vehicles.
Yonsei’s Fluoride-Based Solid Electrolyte Innovation
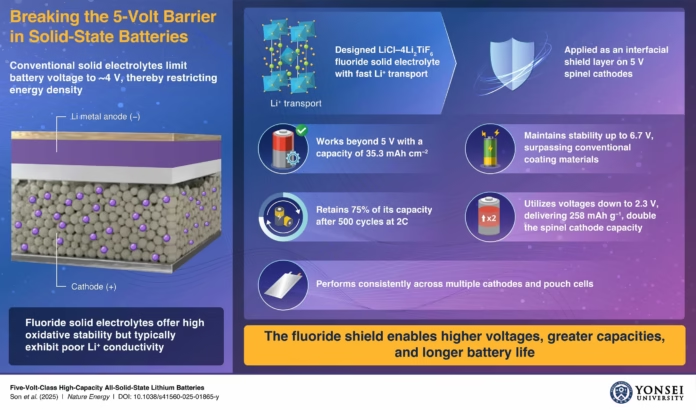
Yonsei University’s research team, led by Professor Yoon Seok Jung, developed a fluoride-based solid electrolyte composed of LiCl–4Li₂TiF₆. This electrolyte exhibits a remarkable Li⁺ ionic conductivity of 1.7 × 10⁻⁵ S/cm at room temperature, ranking it among the highest for fluoride-based materials. Unlike conventional sulfide and oxide electrolytes that degrade beyond 4 volts, Yonsei’s electrolyte remains stable above 5 volts. This innovation enables the use of spinel cathodes like LiNi₀.₅Mn₁.₅O₄ (LNMO), which are known for their high-voltage capabilities but have previously been limited by electrolyte instability. When applied as a protective coating on cathodes, this fluoride shield prevents interfacial degradation, a major cause of battery capacity loss and reduced lifespan, solidifying the battery’s longevity and performance.
Practical Performance in Real-World Applications
The new electrolyte’s impact was demonstrated in pouch-type batteries—the same format used in electric vehicles and consumer electronics—where it retained over 75% of its capacity after 500 charge cycles. It also achieved an ultrahigh areal capacity of 35.3 mAh/cm², setting a new record for solid-state systems. This consistent performance under demanding cycling reveals the material’s practical viability beyond the lab. Moreover, the technology shows compatibility with cost-effective halide catholytes, such as zirconium-based systems, potentially lowering production costs while boosting battery safety and longevity. This breakthrough supports the development of EV batteries that are not only safer but also capable of longer driving ranges and faster charging speeds.
Implications for Electric Vehicles and Renewable Energy Storage
Electric vehicles stand to benefit profoundly from this advancement. The ability to operate batteries at higher voltages directly translates to higher energy density and thus extended driving range—an essential factor driving consumer EV adoption. Furthermore, the enhanced safety and longer cycle life address buyer concerns about battery degradation and fire risks. Beyond EVs, these batteries can support large-scale renewable energy storage systems, which require durable, efficient storage solutions to balance intermittent sources like solar and wind power. By utilizing abundant and low-cost materials, the Yonsei innovation aligns well with global goals to reduce carbon emissions and transition to sustainable energy.
Pioneering a New Paradigm in Battery Design
Yonsei University’s fluoride solid electrolyte represents a paradigm shift in all-solid-state battery design. This technology overcomes the long-standing voltage barrier in solid electrolytes while maintaining exceptional ionic conductivity and compatibility with safe, affordable materials. By addressing critical industry challenges, it enables the next generation of electric vehicles and renewable energy storage to be safer, more powerful, and more affordable. Professor Jung emphasizes that this research is not merely a material breakthrough but a new design rule for future batteries that will power sustainable societies. This breakthrough marks a significant step forward in bridging laboratory innovation and real-world environmental and energy applications.